Preimplantation Diagnosis PGD
What exactly is PGD?
PGD stands for Preimplantation Genetic Diagnosis. It is an advanced genetics test used in conjunction with in vitro fertilization to determine the status of an embryo’s chromosomes. A normal cell has 46 chromosomes in 23 pairs, half from each parent.
If we look at a day 3 embryo cell, in some cases, it may be found to have one extra or one less chromosome. This can cause a child to be born with mental and/or physical deficiencies, such as Down syndrome.
A chromosome abnormality can also prevent the embryo from attaching to the uterus wall, preventing any chance of pregnancy. Or, in the case of many women experiencing Recurrent Pregnancy Loss, it can cause the embryo to stop developing, causing the fetus to miscarry spontaneously.
How likely is it that I may be affected by a chromosome abnormality?
The older you are, the greater the chance that your eggs will be affected. More than 50% of embryos from women aged 35 to 39 have chromosomal abnormalities. For those over 40, the percentages increase to 80% or higher. That’s why older women have far lower pregnancy rates and higher rates of miscarriage.
In addition, patients with Recurrent Pregnancy Loss are believed to produce more chromosomally abnormal embryos regardless of age.
How would PGD improve my chances of a successful pregnancy?
Studies have shown that PGD can:
- double the chance of embryo implantation
- reduce pregnancy loss by as much as three-fold
- increase the likelihood of live births.
Data even found a reduction in miscarriage among IVF patients who did not have recurrent miscarriages, from 23% to 9%.
In women with an average age of 40, they found that the chance of an embryo to implant doubled successfully.
Am I a candidate for PGD?
Women with Recurrent Pregnancy Loss, as well as those who have had problems becoming pregnant, are considered good candidates for PGD.
Free Online Consultation with Dr Thanos Paraschos and his team
How and when is PGD done?
PGD can only be done as part of the in vitro process- when the IVF physician has removed the egg from the female, and it has become fertilized with the male’s sperm before being placed back in the uterus.
After embryos are created in the laboratory, they are grown for 3 days. By this time, embryos contain approximately 8 cells. Each embryo at this point is called a blastomere.
The embryos are placed under a powerful microscope, and very tiny glass instruments are guided to make a small cut in the zona pellucida (a tough outer membrane holding the embryo together). Depending on the health and size of the embryo, one or two cells are taken out. The cut then snaps shut, and no cells can “fall out” accidentally.
Geneticists use a technique called FISH (fluorescence in situ hybridization) to identify the chromosomal makeup of the cell and determine which embryos are most suitable for transfer to the woman’s uterus. In the meantime, during PGD, the embryos develop undisturbed in an incubator.
In some cases, polar bodies, two small cells produced by the ripening egg before fertilization, may be tested. This only provides genetic information from the egg. It will not detect abnormalities that may occur after the egg is fertilized by sperm.
On day 5, the woman returns to EMBIO to discuss her PGD test results. Our medical team will help her decide how many embryos will be transferred into her uterus.
Dr Paraschos participated in the world's first PGD.
Ask Dr Paraschos what you need to know about Preimplantation Diagnosis at EmBIO!
Fluorescence in-situ hybridization (FISH)
PGD testing differs from most genetic testing since it is done on only one or two embryonic cells and must be completed within 48 hours to allow embryo transfer by Day 5. Since standard chromosome analysis takes several days, a different method called fluorescence in-situ hybridization (FISH) is performed.
Each chromosome has unique areas of DNA present only on that chromosome. A small DNA probe is used to recognize these unique patterns and fluoresce or light up when it attaches to the chromosome. Each probe shines a light with a different colour, allowing several chromosomes to be tested simultaneously. This technique is called FISH (fluorescence in-situ hybridization).
EmBIO uses FISH for chromosomes 13, 15, 16, 18, 21, 22, X, and Y because these are the most commonly abnormal chromosomes. A normal cell should show 2 FISH signals (or lights) for each numbered chromosome, and either 2 X signals for a female or 1 X and 1 Y signals for a male. Only five different colours can be used, so most tests are done in two parts. The first 5 chromosomes are tested, those probes are washed off, and the remaining chromosomes are tested. The washing process can affect the integrity of each chromosome; therefore, a maximum of two cycles of FISH are used per cell. For this reason, every chromosome can not be tested.
An example of a regular FISH aneuploidy test showing two copies of each of the tested chromosomes:
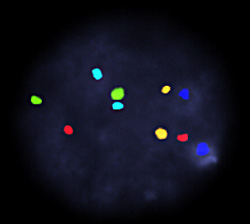
| Color / | Chromosome |
| Red = | 13 |
| Aqua = | 16 |
| Blue = | 18 |
| Green = | 21 |
| Gold = | 22 |
Is PGD 100% accurate?
PGD, the only test available to determine aneuploidy, has an accuracy rate of over 90%. There is a false positive error rate of only 4.7%. Yet to identify a false negative (classifying an abnormal embryo as normal), prenatal testing is still recommended via chorionic villous samples (CVS) or amniocentesis.
Is PGD Safe?
Yes. Data from many years of PGD in animals and approximately 1200 live births in humans indicate that PGD does not increase birth defects or chromosomal disorders. PGD is done before the embryo’s genetic material becomes “active.”
Since it is done so early, the cells inside the embryo are still all identical, and each cell is capable of becoming any part of a baby. Removal of a few of the early embryo cells does not alter that embryo’s ability to develop into a complete, normal pregnancy.
Are there risks to the embryo?
PGD does not affect the normal development of the embryo or fetus. It is estimated that the removal of one cell reduces the ability of the embryo to implant by less than 3%.
Which patients stand to benefit from PGD?
Virtually all couples over 35 without a history of repeated IVF failures are excellent candidates. Studies show that women who have Recurrent Pregnancy Loss, previous aneuploid conceptions, known chromosome abnormalities, and single gene defects can also benefit. It is unclear whether women with repeated IVF failures would be good candidates for the procedure. In any of these problems, the cause may be in the chromosomes of either the man or the woman.
* Read more: Indications for PGD
What is the cost for PGD?
There is an additional cost for PGD above your IVF cycle. However, you may select the “Advanced IVF with PGD”, which includes a PGD test in day-5 embryos. Check the IVF costs at EmBIO.
How are embryos chosen for transfer?
Embryos that have both a normal test result and appearance should be transferred. Sometimes embryos that have normal genetic tests will have a physical problem that prevents them from growing normally. Sometimes embryos that have abnormal genetic tests will appear to be physically normal. The combination of normal genetic testing with a normal physical appearance indicates the highest chance of having a healthy pregnancy.
What physical characteristics are used to determine normal appearance?
Embryologists give a “score” to the embryo based on the uniform size of the different cells, the number of cell fragments present, and other criteria reflecting the physical appearance of the embryo. Embryos that do not have at least 5 cells on day 3 or embryos that are given a poor score rarely succeed in implanting successfully in the uterus.
While embryo morphology helps pick the best embryos for transfer, it is known that many embryos with significant chromosome abnormalities have normal morphology. Even among embryos that successfully reach an advanced developmental stage (blastocyst), 25% are still chromosomally abnormal! Combined with embryo morphology, aneuploidy screening helps select the best embryos for transfer in an IVF cycle.
What if all the healthy embryos are not used?
All decisions about which embryos to transfer and how to use the remaining embryos will be made together with the couple and their doctor(s).
To make an appointment for an initial consultation with one of our reproductive specialists, please call +30 2106774104 or email Dr Paraschos.
What would you like to ask our PGD specialist?
The following questions may help you state your problem to EmBIO’s PGD specialist.
- Are you a woman over 35?
- Have you experienced several miscarriages?
- Have you had a prior pregnancy with a chromosome abnormality?
- Have you experienced several failed IVF cycles?
- Has conceiving been difficult due to a low sperm count?
- Do you or your partner carry a balanced structural chromosome rearrangement?
- Do you have a family history of Huntington’s disease?
- Do you or your partner carry a recessive genetic disease such as cystic fibrosis?
- Do you or your partner carry an X-linked genetic disease such as hemophilia or Duchenne Muscular Dystrophy?
- Do you want to balance the gender in your family (select the gender of your embryo)?
Free Online Consultation with Dr Thanos Paraschos and his team
What should I know about chromosomes?
If you look at a cell under a microscope, you’ll see string-like structures in the cell’s center or nucleus. These are chromosomes that contain DNA- our genetic roadmap. Normal human cells (for adults, babies, fetuses, and embryos) contain 46 chromosomes in 23 pairs, half from each parent.
The condition of having an embryo or zygote with either more or less than 46 chromosomes is called aneuploidy. Very often, the likelihood of aneuploidy increases with the woman’s age, but it can also occur in women under 35.
Aneuploid embryos may have extra (called trisomy) or missing (monosomy) chromosomes. A baby carrying an extra or missing chromosome may be born with mental and/or physical defects. Down syndrome is a common example.
How does aneuploidy affect my ability to conceive or maintain a pregnancy?
A chromosomal abnormality (aneuploidy) can prevent the embryo from attaching to the uterus wall, eliminating any chance of pregnancy. It may also cause the implanted embryo to stop developing, resulting in pregnancy loss.
* Read more: aneuploidy screening
How can PGD help improve my chances of becoming pregnant or carrying to term?
Studies reveal that PGD of aneuploidy:
- increases the chance of implantation
- reduces pregnancy loss
- increases live births.
- data suggest a four-fold reduction in the frequency of chromosomally atypical conceptions after PGD.
Single gene disorders
The term single gene disorder refers to any of the hundreds of inherited diseases caused by a mutation (change) in a single gene.
Common examples include:
- cystic fibrosis
- alpha and beta-thalassemia
- myotonic dystrophy
- sickle cell anaemia
- Duchenne muscular dystrophy,
- fragile x syndrome.
PGD for single gene disorders avoids pregnancy termination.
Preimplantation Genetic Diagnosis (PGD) is now available for virtually all single gene disorders. PGD aims to provide patients at risk of transmitting an inherited disorder to their children the chance to initiate an unaffected pregnancy. PGD dramatically reduces the likelihood that an affected fetus will be detected during prenatal testing and therefore decreases the probability that parents will face the difficult decision of whether or not to terminate a pregnancy.
The single gene PGD service
The single gene PGD program has proven extremely popular, with the number of referrals doubling in the past three years. The methods used are at the cutting edge of genetic diagnosis, rapidly yielding highly accurate results. EmBIO single gene tests generally have 99% or higher assay accuracy rates, with results available within 24 hours of sample receipt (range 5-36 hours).
Unique tests
Every single gene test performed by our lab is designed specifically for the couple requesting PGD. Each protocol considers each individual’s unique genetic makeup, allowing the production of more reliable tests.
In effect, we have the capacity to develop PGD tests for any single gene disorder. PGD tests for single gene disorders are designed specifically for the couple requesting PGD; therefore, extensive preliminary preparation is required. In Vitro Fertilization cycles should not be initiated until the case is reviewed and information regarding specific preparation time is approved.
We offer HLA-typing
In addition to PGD for single gene disorders, we have the ability to perform human leukocyte antigen (HLA)- typing of embryos.
There has been a growing interest in HLA- typing of embryos as it allows parents to conceive a child that is capable of providing histocompatible stem cells to save a life of a sibling with a disease. The stem cells are obtained from umbilical cord blood at the time of birth and then transferred to the affected sibling. This approach has been used to cure children with various forms of inherited anaemia and children with leukaemia.
Key features of the gene PGD service
- PGD can help parents avoid having to contemplate pregnancy termination
- Available for virtually all single gene disorders
- Diagnostic protocols tailored to individual patients
- Extremely high assay accuracy rates
- Employs analysis of hypervariable polymorphisms for accurate detection of contamination
- Rapid results
- HLA – typing available
Karyomapping, a revolutionary genetic test
Scientists developed a revolutionary test that will allow future parents to test their embryos for almost all known genetic diseases. Karyomapping is based on the same procedure as PGD (the same doctor developed it). Still, it can detect all known genetic diseases in embryos within a few weeks (the only exceptions are those that appear suddenly due to an accidental mutation).
Innovation and new services
At our unit, we continually strive to extend the services offered to our clients. We will be offering novel technologies in the near future.


